By Meghan McCarthy
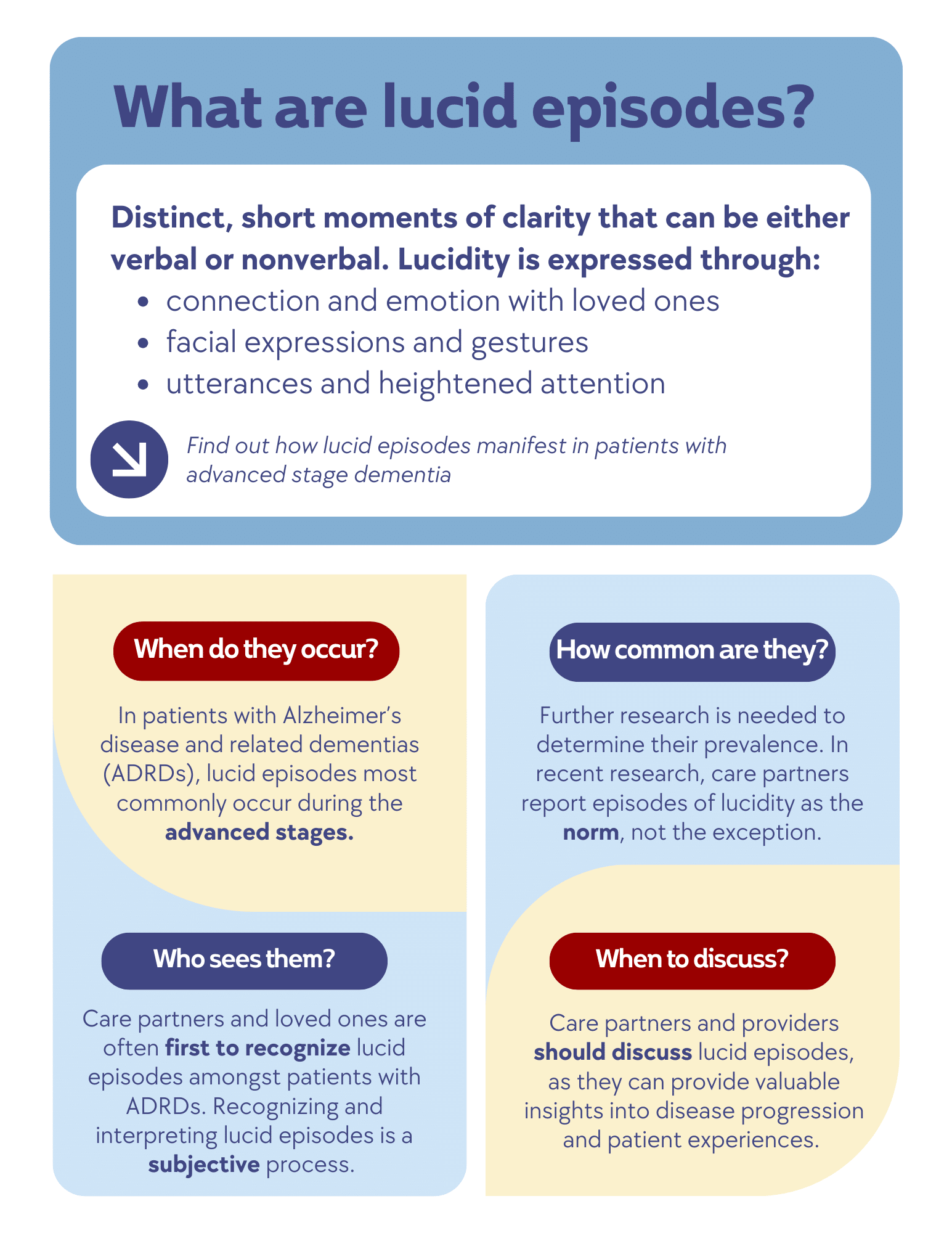
Lucid episodes in dementia describes moments of unexpected clarity in individuals with Alzheimer’s disease and related dementias (ADRDs). These episodes, which may be verbal or nonverbal, can evoke deep emotional responses from caregivers and other loved ones. Experts, Jason Karlawish, MD, co-director of the Penn Memory Center, and Justin Clapp, PhD, MPH, assistant professor of Anesthesiology and Critical Care and Medical Ethics and Health Policy at the Perelman School of Medicine, discuss the nature, frequency, and implications of these episodes.