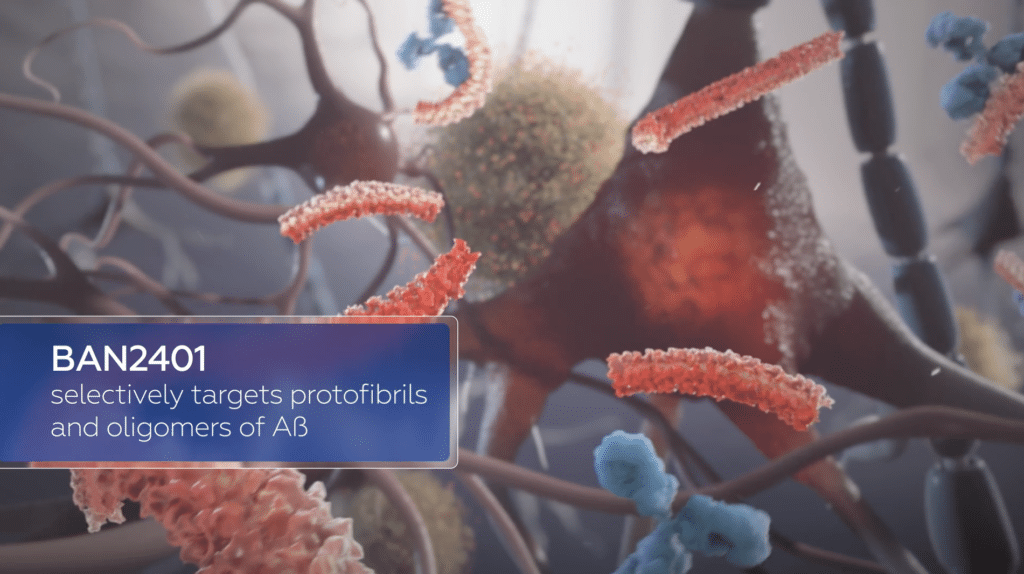
A screen capture from a BioArctic video describing the mechanism of BAN2401, or lecanemab.
Drugmakers Eisai and Biogen announced yesterday the experimental drug lecanemab helped slow cognitive decline in people living with Alzheimer’s disease (AD).
“This is an extremely encouraging development,” said Penn Memory Center (PMC) Co-director David Wolk, MD. “The observed reduction in decline may reflect meaningful differences in function for a significant portion of patients.” More details are needed to evaluate the drug confidently, he added.
PMC Co-Director Jason Karlawish hopes that the announcement marks a pivotal milestone in Alzheimer’s disease history, but clarifies that his hope is based on a press release.
“Science relies on the open and unbiased exchange of information, and three key means to achieve this are publishing results in a peer-reviewed journal, presenting the results to open scientific forums with ample time for discussion and debate, and making the data available for confirmatory analyses,” he said. “I look forward to these events.”
In the phase 3 clinical trial of lecanemab, the drug slowed cognitive decline by 27 percent over 18 months. About a fifth of participants experienced side effects such as brain swelling.
“There are very real side effects with this class of drug, but it does seem an important first step to effectively treating the biology of AD,” said Dr. Wolk.
People with AD have a build-up of a particular protein, amyloid-beta, in their brains. This leads to the death of brain cells. Lecanemab is a human monoclonal antibody that targets this amyloid-beta build-up.
“We have moved the needle,” said Edward Lee, MD, PhD, Co-Associate Director of the Alzheimer’s Disease Research Center at the University of Pennsylvania, “and this brings tremendous hope that our continued efforts to research and understand AD and related dementias will continue to benefit the patients, families, and caregivers affected by AD.”
Lecanemab will be reviewed by the Federal Drug Administration (FDA) this year. A decision on whether to approve the drug is expected in January 2023, and coverage decisions will follow.
Read the Eisai press release here. Read complete coverage in The New York Times.

PMC Co-Director and Director of the Alzheimer’s Disease Research Center
This is an extremely encouraging development, that this phase 3 study met not only its primary endpoint but also all its secondary endpoints, encompassing several measures of both cognition and function. While the effect was modest, the disease change over 18 months is relatively slow without treatment, and the observed reduction in decline may reflect meaningful differences in function for a significant portion of patients. It is also possible that these benefits may increase over longer time frames, but that will need to be studied in the future.
Of course, we have seen only these top-line results and will need to hear more details of the findings to evaluate confidently. Notably, the description of effect size is similar to reports of other drugs within this class, which does further support some movement in the disease course. Nonetheless, this is not a home run and there are very real side effects with this class of drug, but it does seem an important first step to effectively treating the biology of AD.
As for what this means for our patients, the drug will be reviewed now for full FDA approval, in addition to accelerated approval, for which its earlier phase data were being considered. Coverage decisions will follow, and these will be important steps before the drug will be used in clinical practice. I think this brings hope that this and similar drugs, two of which will report their phase 3 results in the next six months, will become an important addition to our currently limited treatments for AD.

PMC Co-Director and Co-Associate Director of the Alzheimer’s Disease Research Center
In the history of Alzheimer’s disease, I hope that Tuesday, September 27th, 2022 will become one of the great events. This is the day when the pharmaceutical companies Eisai and Biogen announced the “topline results” of CLARITY AD. They report that after 18 months of treatment with lecanemab or placebo, comparing persons who received placebo to persons who received the drug showed the drug caused slower declines in cognition and day-to-day function.
The companies will apply to FDA and other countries’ regulatory authorities for review of these data, and, they hope, approval to market the drug for the treatment of Alzheimer’s disease. If the regulatory authorities agree with Eisai and Biogen, CLARITY AD will be remembered as the study that let the United States achieve goal No. 1 of our National Alzheimer’s Plan: to prevent and effectively treat Alzheimer’s disease by 2025.
The source for my hope is a press release, not a peer-reviewed, scientific paper, or presentation to an FDA advisory committee or scientific meeting. Science relies on the open and unbiased exchange of information, and three key means to achieve this are publishing results in a peer-reviewed journal, presenting the results to open scientific forums with ample time for discussion and debate, and making the data available for confirmatory analyses. I look forward to these events.

Co-Associate Director of the Alzheimer’s Disease Research Center
I am very encouraged by these results. While there are potential side effects in some individuals and the overall clinical effect is modest, these results reaffirm that research can improve the human condition, even for difficult diseases such as Alzheimer’s disease.
This trial represents the work of countless researchers, from the pathologists who first described the accumulation of plaques in AD brains, to the biochemists who purified beta-amyloid, geneticists who identified mutations that cause amyloid, neuropsychologists who developed the tools to measure cognition, clinical chemists and radiologists who developed AD biomarkers, clinicians who designed clinical trials and treat persons with dementia, and many others.
We have moved the needle, and this brings tremendous hope that our continued efforts to research and understand AD and related dementias will continue to benefit the patients, families, and caregivers affected by AD.