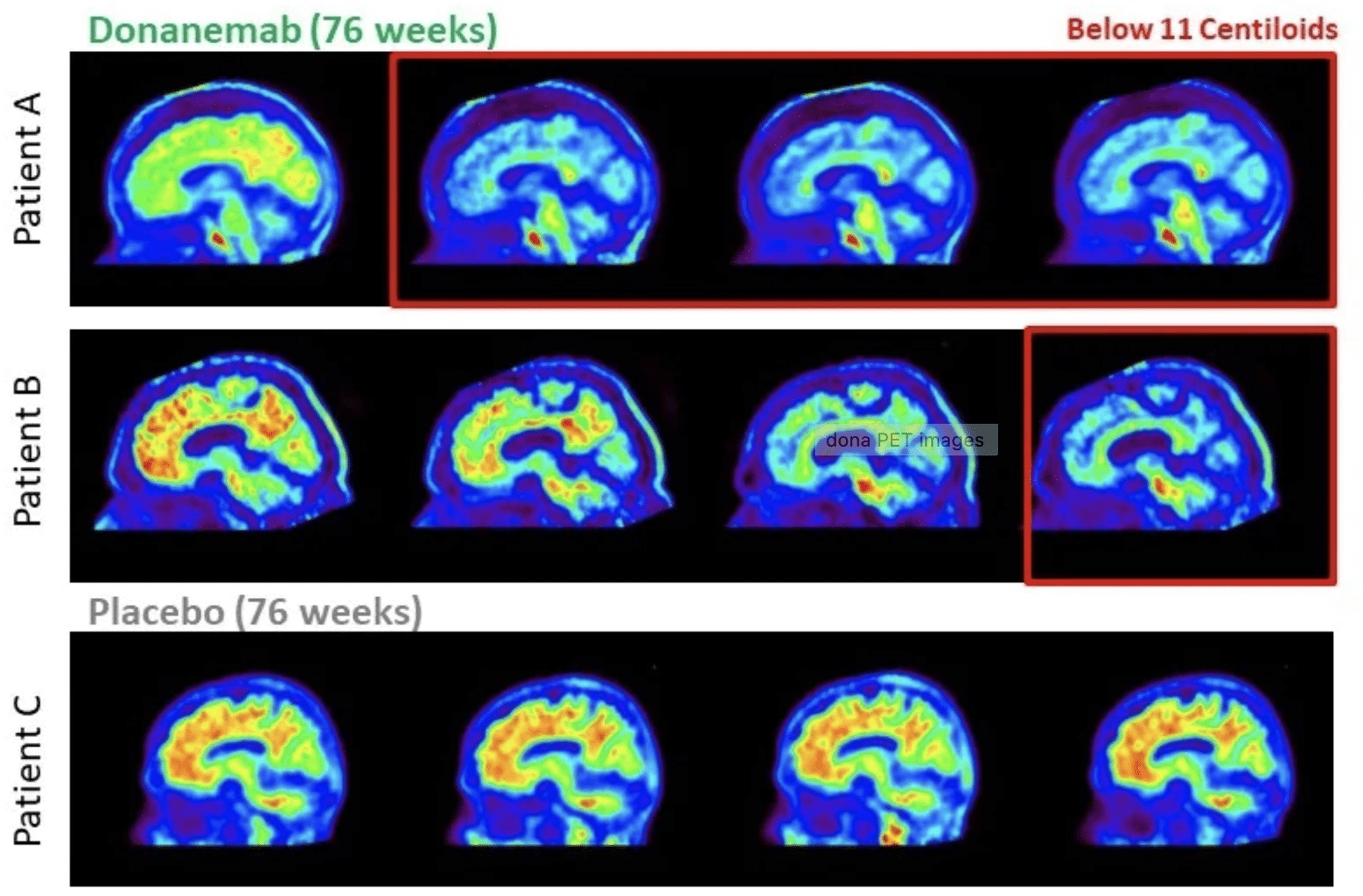
Brain scans show clearance of amyloid plaques, shown in red, in two patients, A and B, who received donanemab, compared to higher levels of amyloid in a patient who received placebo (bottom) in Eli Lilly’s trial.
The Food and Drug Administration (FDA) has postponed its decision on the Alzheimer’s drug donanemab, which was expected by the end of March. Instead, the FDA will ask an independent panel of experts to review questions about the drug. Eli Lilly and Company, the drug’s manufacturer, confirmed this development.
The FDA’s request for an advisory committee follows a longer than expected wait for a decision that was expected by the end of 2023 and then sometime in the first quarter of 2024.
The published and presented data on donanemab describe a drug that’s much like lecanemab, an effective treatment for persons with mild cognitive impairment or mild-stage dementia, which is dispensed by infusion every two weeks. Donanemab was attractive to some patients because it required a monthly infusion.
“This is a surprise, but it’s not a cause for worry; the data show the drug’s effective,” said Penn Memory Center Co-Director Jason Karlawish, MD. “Most likely, FDA will ask the committee to weigh in on how to translate the study’s design into the FDA guidance for how to prescribe it.”
He noted the study of donanemab enrolled persons with certain levels of tau protein in their brain, and that the drug was discontinued after amyloid was cleared. Tau is one of the pathological hallmarks of Alzheimer’s disease.
“Neither of these design features is part of how we prescribe lecanemab. Should they be for donanemab? And if so, why not lecanemab? Those are great questions for an advisory committee to weigh in on,” Dr. Karlawish said.
The PMC will provide updates on the timing of the hearing and its outcomes.