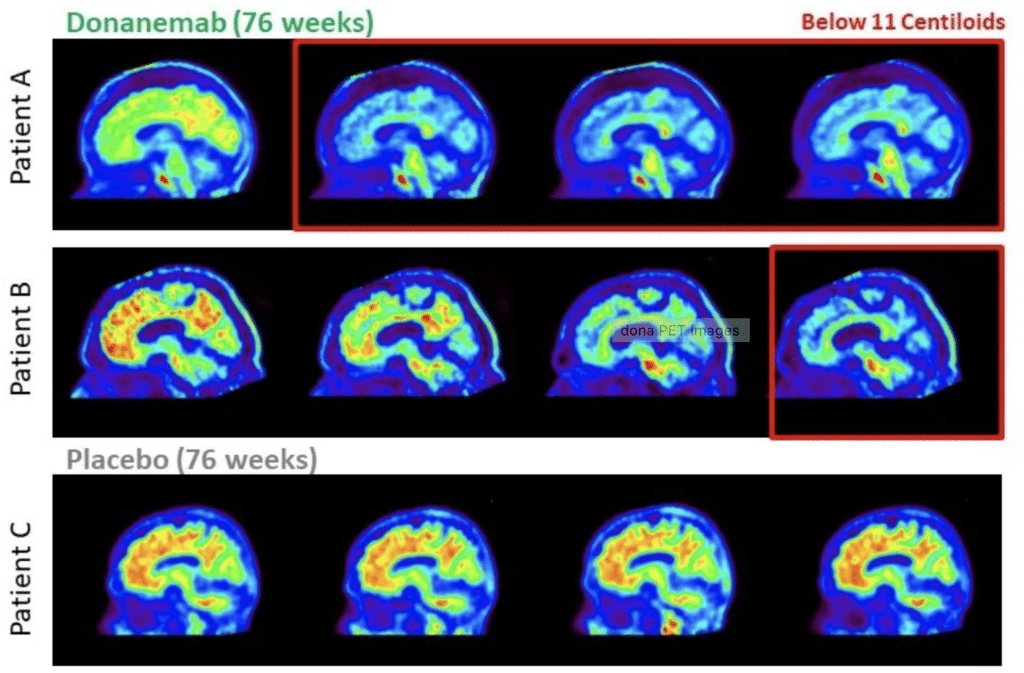
Brain scans show clearance of amyloid plaques, shown in red, in two patients, A and B, who received donanemab, compared to higher levels of amyloid in a patient who received placebo (bottom) in Eli Lilly’s trial. (time.com)
By Caitlin Kearney
The investigational drug donanemab was found to slow cognitive decline in people living with early-stage Alzheimer’s disease, Eli Lilly announced this morning.
In the TRAILBLAZER-ALZ 2 study of 1,700 participants, including at the Penn Memory Center, donanemab slowed cognitive decline by 35 percent in people who received the drug compared to those taking a placebo.
“This is the first Phase 3 trial of any investigational medicine for Alzheimer’s disease to deliver 35 percent slowing of clinical and functional decline,” said Daniel Skovronsky, MD, PhD, Eli Lilly’s chief scientific and medical officer.
Donanemab carries the risk of brain bleeding and swelling, known as ARIA. About 2 percent of study participants experienced serious ARIA.
Drs. Jason Karlawish and David Wolk, PMC co-directors, said they look forward to a formal presentation of these results in published research and at a conference in late July but offered that this could be “a true inflection point for the field.”
They draw three clear lessons from this announcement, particularly following the success of Biogen’s lecanemab:
- The general approach of targeting and effectively clearing amyloid alters clinical outcomes.
- Disease stage matters, and earlier treatment is more effective.
- A precision medicine approach to Alzheimer’s disease is vital.
Penn researchers are particularly interested in learning more about the strategies for dosing and stopping donanemab, which could make this a more practical and less expensive option for treatment in the future.
Once PMC researchers have had a chance to access and review more data, a community discussion will be held for patients and families.