Kisunla (donanemab) is a treatment to slow the progression of early symptomatic Alzheimer’s disease. This guide will help you understand the risks of Kisunla, focusing on brain swelling and bleeding (known as Amyloid-related imaging abnormalities or ARIA) and infusion-related reactions.
What is ARIA?
Amyloid-related imaging abnormalities (ARIA) are changes seen on brain MRI scans. There are two types:
- ARIA-E (edema): brain swelling
- ARIA-H (hemorrhage): small (microscopic) brain bleeding
ARIA is a side effect of anti-amyloid therapies like Kisunla, which help remove one of the proteins that causes Alzheimer’s disease – amyloid – from the brain. Though microscopic brain bleeding can also occur naturally in Alzheimer’s disease, anti-amyloid therapies increase this risk.
Symptoms of Brain Swelling or Bleeding
Brain swelling or bleeding usually does not cause symptoms and resolves in about 10 weeks. When symptoms occur, they’re usually mild, such as headaches or increased confusion. In rare cases, symptoms can be more severe, such as seizures.
Who is at Risk?
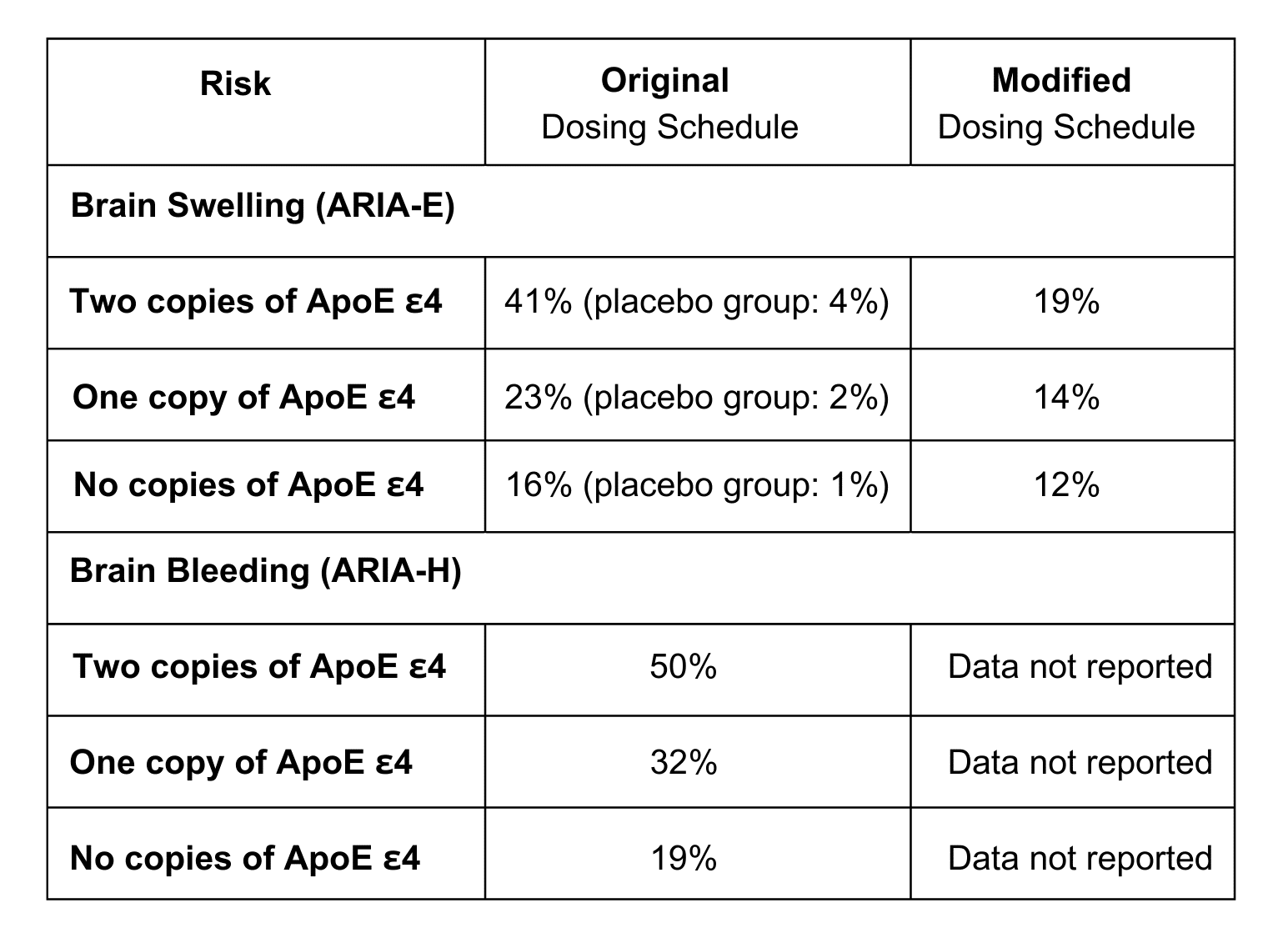
The risk of brain swelling and microscopic bleeding depends on genetic factors, particularly the presence of the ApoE ε4 gene. This gene also increases the likelihood of developing Alzheimer’s. Penn Medicine patients who are candidates for Kisunla will be tested for ApoE ε4 to understand their risk of developing ARIA.
Monitoring for Side Effects
Patients on Kisunla will undergo regular monitoring to look for signs of brain bleeding and swelling:
- MRI scans: At 4 weeks, 8 weeks, 12 weeks, 6 months, 1 year, and 18 months of treatment.
- Symptom monitoring: Regular follow-ups to monitor for new symptoms.
Clinical Trial Findings on ARIA
Two clinical trials have described how often swelling (ARIA-E) or microscopic bleeding (ARIA-H) happens with Kisunla treatment. The first trial followed the original dosing schedule, while the second used the modified dosing schedule—currently implemented at the Penn Memory Center—which features a slower titration. This adjustment reduces the risk of brain swelling (ARIA-E) while maintaining the overall risk of brain bleeding (ARIA-H) at 20% across all patients.
TRAILBLAZER-ALZ 2 Trial
- Participants: 1,736 (860 on Kisunla, 876 on placebo)
- Study length: 18 months
- Note: Used the “original dosing schedule” (see below)
- Findings: Higher rates of ARIA in those with the ApoE ε4 gene
TRAILBLAZER-ALZ 6 Trial (ongoing)
- Participants: 842
- Study length: 24 weeks, with evaluations at 12 and 18 months
- Note: Using a “modified dosing schedule” (see below)
- Findings: A lower rate of ARIA compared to original dosing
Infusion-Related Reactions
In the TRAILBLAZER-ALZ 2 trial, 8.7% of participants experienced infusion-related reactions such as chills, sweating, irritation of skin, headache, nausea, chest pain, vomiting, or problems breathing. These typically occurred within 30 minutes of the end of the infusion and between the second and fifth infusion. Anaphylactic reactions (a severe, life-threatening allergy) occurred in 0.4% of participants.
Risk Based on ApoE ε4 Gene